Pharmacovigilance rules updated by ANVISA
The Brazilian National Health of Surveillance Agency (ANVISA) published on July 29, 2020 the Resolution RDC No. 406/2020 and Normative Instruction – IN No. 63/2020 in order to update the regulations on pharmacovigilance. The new regulatory framework is the result of an extensive discussion between ANVISA and the industry through public consultations.
Both RDC No. 406/2020 and IN No. 63/2020 were unanimously approved at the 12th Public Meeting of the ANVISA Board, held on July 21.
RDC No. 406/2020 inaugurates a new regulatory framework and establishes the Good Pharmacovigilance Practices, revoking Resolution RDC No. 4/2009 – formerly responsible for establishing the industry standards. Meanwhile, IN No. 63/2020 deals with the Periodic Report of Benefit-Risk Assessment (RPBR) that must be submitted to ANVISA, and updates the former IN No. 14/2009.
We understand that post-marketing monitoring practices have been formalized and/or improved. Find below some of the amendments:
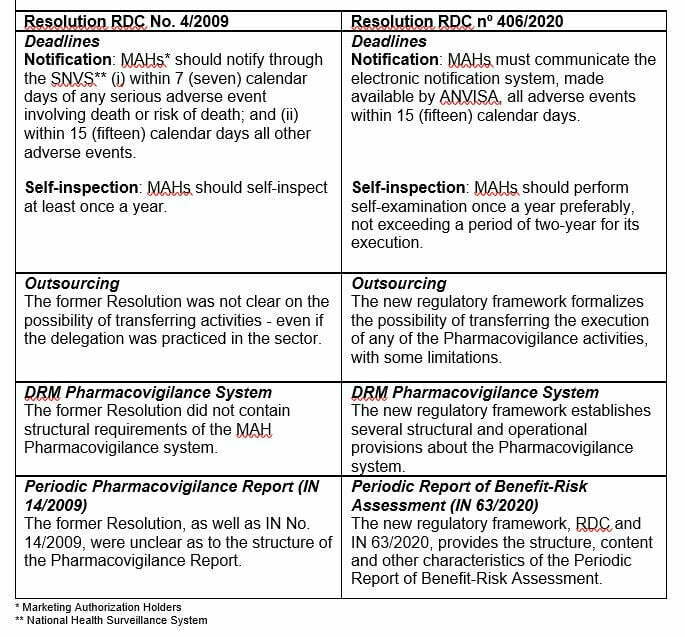
Resolution RDC No. 406/2020 and Normative Instruction No. 63/2020 also update other aspects that are very interesting to the sector and should be evaluated with a great deal of discretion.
Resolution RDC 406/2020 and Normative Instruction No. 63/2020 will become effective in 90 days counted from the publication date.


